Technical resources >> Green Chemistry and chemical process improvement >> Green Chemistry >> Benefits of Green Chemistry
Benefits of Green Chemistry
Despite significant improvements in efficiency and process safety, the design and commercial production of chemical products pose numerous challenges:
- Chemistry: non-ideal conversion, competing reactions, hazardous chemicals, raw material impurities, etc.
- Engineering: undesirable by-products, solvent recovery, waste treatment, energy transfer, hazardous reaction conditions, etc.
>>>> Green Chemistry addresses these holistically
Chemical products are the building blocks of innovation in many parts of the manufacturing economy.
Green Chemistry improves the economic position of businesses through:
- Higher material efficiency
- Lower pollution intensity and waste treatment costs
- Safer ingredients, processes and products
- Lower risk to health and of accidents
- Sustainability benefits providing more added-value to customers
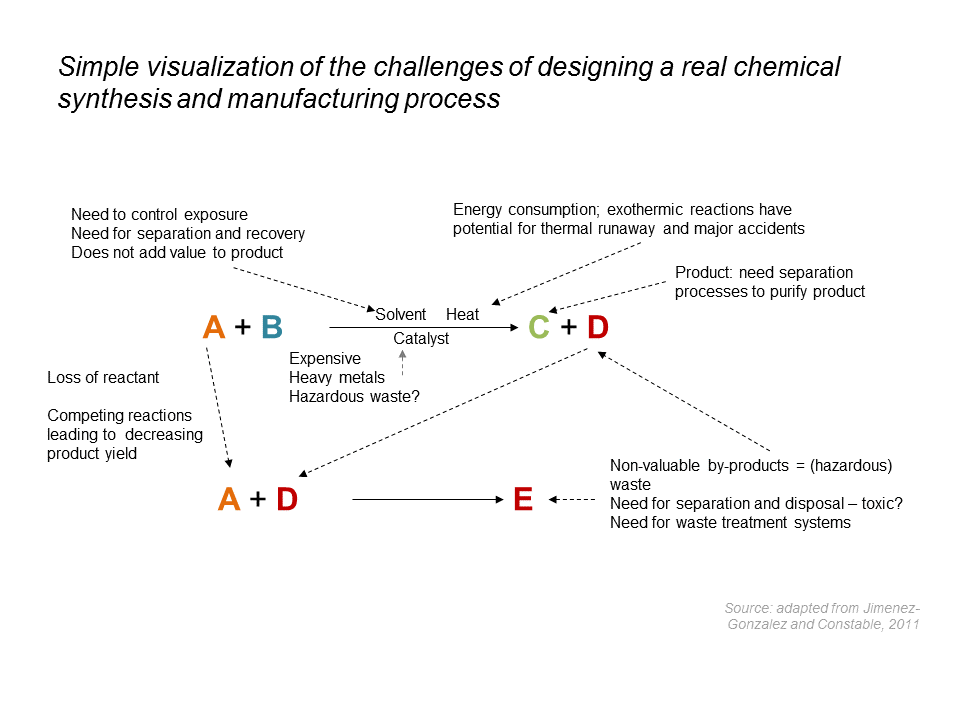
Comment to the image above:
Thermal runaway: Chemical reactions either release heat (exothermic) or absorb heat (endothermic). The majority of chemical reactions (i.e. synthesis processes) carried out in industry are exothermic. In some cases, an exothermic reaction can lead to a thermal runaway if the rate of heat generated by the reaction exceeds the removal rate. As the surplus heat begins to raise the temperature of the reaction mass, the rate of reaction starts to increase. This in turn accelerates the rate of heat production. As a general rule of thumb, a 10 °C increase in temperature roughly doubles the rate of reaction and thus heat release resulting in an exponential increase in temperature (and possibly pressure). Whereas, the rate of heat removal is generally linear. As soon as the heat production exceeds the heat removal rate, the process will be a thermal runaway and may result in large increases in temperature and pressure leading to a major explosion.
